Uncovering the Secrets of Mice and Human Genomes
Written on
Chapter 1: Evolutionary Insights into Mice and Humans
Mice and humans diverged in their evolutionary path around 75 million years ago, during the age of the dinosaurs. Surprisingly, the genetic makeup of both species is nearly identical, with the real distinction lying in the regulation of genes—specifically, how and when these genes are activated or silenced. The Encode international consortium, dedicated to mapping DNA elements, has made significant strides in elucidating these regulatory mechanisms for both species, enhancing our understanding of their differences and aiding in the use of mice as model organisms for biomedical research.

In 2002, the sequencing of the mouse genome led to a groundbreaking revelation: mice and humans possess nearly all the same genes. Notable exceptions exist, particularly within the immune system, which has evolved to combat different pathogens in each species. Other differences are seen in genes responsible for mouse-specific traits like tail formation. Thus, the focus shifted from the genes themselves to their regulatory mechanisms, culminating in the establishment of the Encode project in 2003 by the National Institutes of Health (NIH).
A gene comprises a sequence of DNA that encodes the instructions for producing a protein. The activation of these genes depends on nearby sequences called promoters and distant regulatory elements known as enhancers. The Encode consortium has meticulously mapped these regulatory elements in both the human and mouse genomes, and their findings have been published in multiple prestigious journals.
The database generated by Encode serves as a valuable resource for researchers globally, promoting advancements in biomedical studies. Preliminary conclusions reveal that the sequences of regulatory elements have significantly changed over the 75 million years of evolution separating mice from humans. These elements do not independently control gene activity; rather, they interact with transcription factors—proteins that facilitate gene expression. Interestingly, about half of the transcription factor binding sites in mice have no equivalent in humans, while a quarter have shifted positions over time. Notably, enhancers show greater divergence than promoters.
Section 1.1: The Role of Transposons in Genetic Regulation
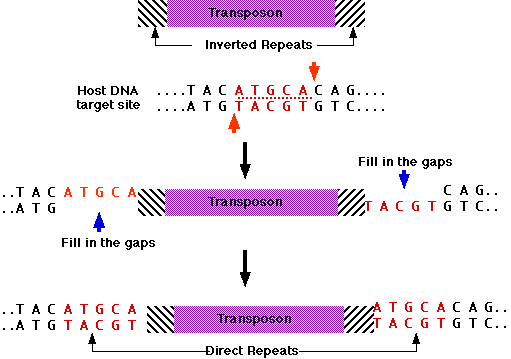
Another vital aspect of this research involves transposons—DNA segments that can relocate within the genome. These elements often carry regulatory functions, and their movement can drastically influence gene regulation. The Encode consortium has identified that many transposons exhibit species-specific regulatory roles, challenging the traditional view of them as mere "parasitic DNA." Instead, they contribute significantly to the regulatory landscape of the genome, providing unexpected flexibility in gene regulation.
The concept of regulatory plasticity, highlighted by researchers, aligns with the earlier work of Barbara McClintock, who discovered transposons in the 1940s. Her groundbreaking research demonstrated that these mobile elements play crucial roles in gene regulation and organismal development. The findings from Encode reinforce her theories and provide further evidence of the dynamism inherent in genetic regulation.
The differences in gene regulation between mice and humans can be attributed to the variability of these regulatory elements, whether due to transposons or other factors. Surprisingly, many genes exhibit activity levels that are species-dependent, rather than organ-specific. For instance, while a mouse liver may not closely resemble a human liver, the intricacies of its gene regulation reveal significant insights into their functional differences.
Numerous genetic variations associated with human diseases have corresponding elements in the mouse genome, particularly within regulatory areas of chromatin. This finding underscores the value of using mice as a model for investigating genetic predispositions to human diseases.
Scientists believe that their discoveries will prompt a reevaluation of the relationship between evolutionary conservation and genomic functionality. Regulatory regions can adapt and evolve more freely than coding sequences, allowing for varied genomic expressions.
Ultimately, the Encode project's central goal is to enhance the efficacy of mouse models in drug research, establishing them as premier subjects for biomedical investigations—a notion once deemed implausible.
Chapter 2: Practical Applications of Mouse Models in Research
The first video titled "Mice, Rats, Mice, Beavers, Mice, Squirrels, and More Mice..." explores the diverse world of rodents, highlighting their significance in scientific research and the insights they provide into various biological processes.
The second video, "How To Get Rid of Mice | EVERYTHING You NEED to Know," offers practical advice on managing mouse populations, connecting the dots between their biological traits and implications for human environments.