Fascinating Journey Through Genetic Discoveries and the Eyeless Gene
Written on
Chapter 1: Unraveling the Eyeless Gene
The exploration of our biological essence through genetics is a captivating and intricate narrative. To delve into this multifaceted story, we can focus specifically on the discovery of a significant gene that governs the formation of our eyes—essentially shaping how we perceive the universe around us. This is the tale of the eyeless gene.
In January 1915, amidst the backdrop of the Great War, a newly graduated Ph.D. geneticist named Mildred Hoge from Columbia University was teaching zoology at Indiana University. She made a groundbreaking announcement in the American Naturalist, identifying a novel gene in fruit flies, which she aptly named "eyeless," due to the striking phenotype of the mutation that resulted in the absence of eyes. Not only did Hoge discover this unique mutant, but she also successfully bred the delicate eyeless flies, quantitatively analyzing how this mutation was inherited and pinpointing its location on the fourth chromosome of the fruit fly.
In December 1919, Mildred married Aute Richards, who would go on to lead the Zoology department at the University of Oklahoma. Unfortunately, due to nepotism regulations, Mildred faced challenges in securing a position there until she finally obtained an associate professorship shortly before her retirement in 1948.
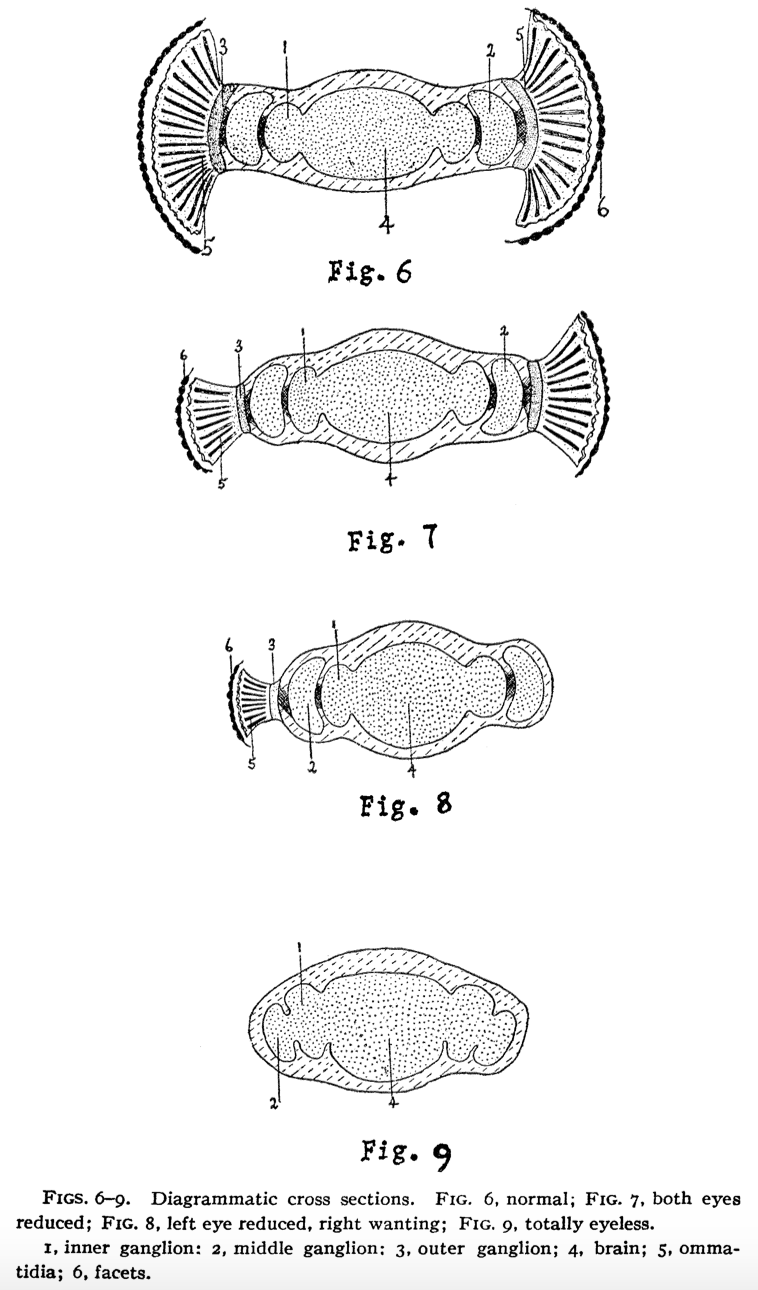
In 1925, she published a comprehensive anatomical study comparing eyeless flies with their normal counterparts, motivated by an inquiry from Thomas Hunt Morgan, a Nobel laureate and her doctoral advisor. Morgan cited several of her papers in his influential work, The Mechanism of Mendelian Heredity, indicating a strong collaborative relationship between them.
Hoge Richards addressed Morgan's question by documenting that the mutations from the eyeless gene exhibited varying degrees of impact, ranging from reduced eye structures to complete absence. In flies with diminished eye structures, the optic tract remained intact, while those completely lacking eyes showed significant anatomical changes. This research established the scope of influence the eyeless gene had on eye development—an influence that would take decades to fully comprehend across various species.
The first video explores the scientific discoveries of the 21st century, emphasizing the role of DNA and genetics in understanding life.
Section 1.1: The Eyeless Gene in Higher Organisms
By the early 1940s, Herman B. Chase at the University of Illinois contributed further insights into the inheritance and variability of the eyeless phenotype in mice, proposing the designation ‘ey’ for the gene responsible in mice, aligning with the fruit fly nomenclature.
In subsequent years, the fruit fly, Drosophila melanogaster, became a cornerstone of genetic research. Various mutant strains, including the eyeless strain developed by Hoge, laid the groundwork for genetic investigations. The fundamental mechanisms in simple organisms such as flies serve as valuable models for deciphering similar processes in more complex beings, including humans. Despite the contrasting appearances of fly and human eyes, their shared evolutionary history significantly outweighs these differences.
The utilization of Drosophila in groundbreaking research during the 1970s and 1980s by Christiane Nusslein-Volhard and Eric Wieschaus led to vital discoveries regarding genes essential for embryonic development. Their collaboration, sparked by a brief meeting in Walter Gehring's lab, resulted in the identification of numerous genes pivotal for developmental processes, many of which were later found to be conserved in human development as well.
In the mid-1980s, Markus Noll’s lab in Basel, Switzerland, published findings indicating that specific domains within some of the developmental genes identified by Nusslein-Volhard and Wieschaus were conserved across related genes. One such domain was termed the paired box, abbreviated to Pax, though its exact function remained elusive at that time.
The second video offers a crash course on the history of science, particularly focusing on the role of the gene in evolution.
Section 1.2: Dissecting the Eyeless/Pax6 Gene
In 1989, Noll’s lab published crucial findings demonstrating that Pax-type genes containing the paired-box domain were present in various species, from nematodes to humans. This exploration may seem tangential to our focus on eye development until 1991, when a human gene linked to aniridia—characterized by the absence or reduction of the iris—was discovered to encode a protein containing a paired box domain, identified as a Pax gene.
This gene, responsible for eye development, was named Pax6. Remarkably, Pax6 exhibited such strong conservation across species that only one amino acid difference exists between the mouse and human Pax6 proteins out of 422.
In 1994, Walter Gehring's lab published findings confirming that the eyeless gene in fruit flies is homologous to Pax6 in various animals, including mice, rats, quail, and zebrafish. However, a critical question lingered: while these genes had been identified by their mutant forms, could we confirm that eyeless (or Pax6) genuinely regulates eye development? Despite decades of research, the answer was still unclear.
Chapter 2: Unveiling the Master Switch
One experimental approach to ascertain a gene's function involves expressing it in an atypical location. In 1995, Gehring’s lab conducted an experiment where they expressed Pax6 in unexpected areas, such as the wings and legs of fruit flies. The outcome demonstrated that an eye developed in these ectopic locations, confirming that Pax6 indeed regulates eye development.
Following this, additional studies in more complex organisms affirmed these findings, leading to further exploration of how Pax6 orchestrates eye development. Research by Czerny and Busslinger in 1995 revealed that Pax6 binds to specific DNA sequences and forms dimers that interact with inverted repeat sequences, elucidating its regulatory role.
In 2001, Marquardt et al. established that Pax6 functions as the master switch for the development of retinal cells in vertebrates. Furthermore, studies in 1997 and 2001 highlighted that Pax6/eyeless in Drosophila regulates the expression of rhodopsin 1, a crucial molecule for vision across many organisms.
Section 2.1: Pax6 as a Transcriptional Regulator
The early discoveries surrounding the eyeless mutations across various species revealed that a gene controlled eye development—now recognized as Pax6. This highly conserved gene plays a direct role in the formation of eye-specific cells, including those in the retina. Notably, Pax6 acts as a transcription factor, regulating which genes are activated or silenced during eye development.
Transcription factors like Pax6 act as switches, signaling when and where to initiate the transcription of genes from DNA to RNA. These intricate networks of transcription factors create a cascading effect, ultimately controlling the expression of genes necessary for retinal cell formation.
Thus, Pax6 emerges as a crucial transcriptional regulator that dictates the activation of genes essential for eye development. Mildred Hoge would undoubtedly take pride in her foundational role in this remarkable story.
