Reversing Age-Related Memory Loss through Brain Cell Support Structures
Written on
Chapter 1: Understanding Age-Related Memory Changes
As we grow older, our minds accumulate countless memories, reflecting the rich tapestry of our experiences. However, these memories often lose their sharpness and clarity over time. Despite our occasional forgetfulness about the fickleness of memory, the aging process undeniably affects our cognitive function.
The brain is not immune to the passage of time. Beyond neurological diseases such as dementia, there are fundamental changes that occur in our brains as we age. Key indicators of brain aging include a reduction in volume, an increased risk of strokes, and a propensity for lesions to develop within the brain’s white matter. Additionally, there is a notable shift in cognitive abilities: while fluid intelligence diminishes, crystallized intelligence tends to endure longer, increasingly dominating our cognitive resources.
Memory also undergoes transformation. Although there is a general decline in memory capabilities, certain types show more resilience. For instance, semantic memory—encompassing general knowledge and facts—remains largely intact with age. In contrast, episodic memory (recalling specific events), short-term memory (like remembering a phone number for a brief period), and working memory (holding information temporarily for tasks) tend to deteriorate. Interestingly, the oldest memories, often those formed during adolescence, tend to remain the most vivid over time.
Perineuronal Nets and Memory Function
A multitude of factors likely contribute to age-related memory decline, including DNA damage, chronic inflammation, and decreased mitochondrial efficiency. A notable component of this decline may be linked to perineuronal nets.
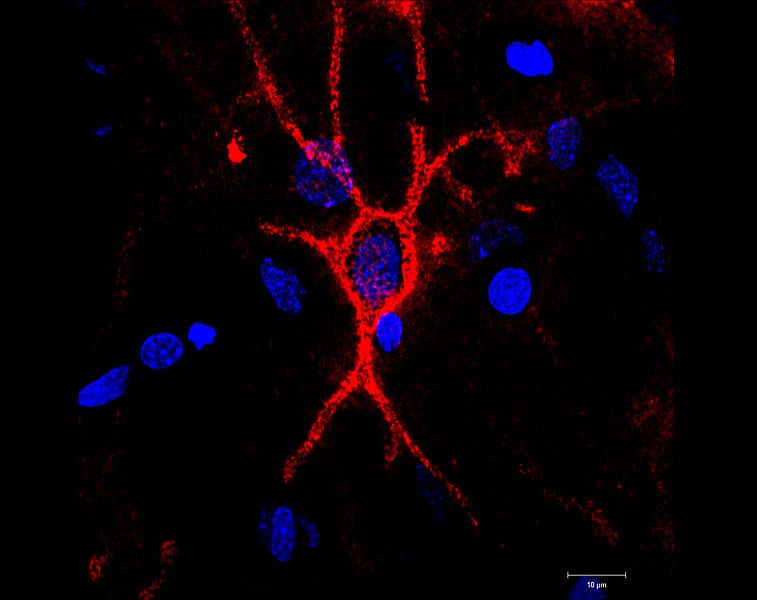
Discovered in 1893, these supportive protein structures—known as chondroitin sulfate proteoglycans—were initially thought to merely encase brain cells. Interest in them resurged in the 1990s when researchers recognized that their composition alters with age. Recent studies on mice indicate that these changes are associated with memory impairments.
Just as a corset restricts movement, perineuronal nets, while protective, hinder the growth and plasticity of neurons. The two primary types of chondroitin sulfate that comprise these nets are C6S and C4S. C6S promotes axon growth and neuroplasticity—think of it as a silk corset—while C4S does the opposite, serving as a steel corset.
With aging, the balance shifts: levels of C4S increase while those of C6S decrease. This phenomenon has been documented in studies involving rodents and chickens, although data on humans remains sparse.
Researchers established that older mice performed worse on memory tests. Subsequent experiments revealed that:
- Mice engineered to produce less C6S relative to C4S exhibited early memory decline.
- Administering chst3 (an enzyme that enhances C6S production) via a viral vector successfully restored both memory function and neuroplasticity in older mice and those genetically predisposed to memory loss.
The authors concluded that this study illuminates a mechanism behind memory loss in aging brains and highlights the potential for treatments targeting perineuronal nets to alleviate age-related memory deficits.
Another intriguing finding suggests that a C4S antibody can enhance memory in mice suffering from neurodegenerative disorders, as well as improve performance in normal mice.
While these results are promising, it is essential to remember that findings in mice do not directly translate to humans, and the path from animal studies to human applications is long and complex. Nevertheless, researchers are optimistic about further developments, as perineuronal nets represent a wealth of potential therapeutic avenues.
As any good goalkeeper knows, maintaining a clean net is essential for success.