The Origins of Chemistry: From Elements to Aristotle's Ideas
Written on
Chapter 1: Understanding Context in Chemistry
The inception of chemistry is a fascinating journey that often feels alien to our modern understanding. To illustrate this, consider the challenge of balancing a checkbook in binary or hexadecimal systems. If you're unfamiliar, these are alternative numeral systems: binary uses only 1s and 0s, while hexadecimal incorporates a broader range of symbols. Our everyday calculations are rooted in the familiar base ten system, which makes it challenging to switch our perspective.
This analogy highlights how deeply ingrained our current understanding of atoms is, making it hard to grasp that the concept of atoms wasn't widely accepted until around 1860—merely 160 years ago! For over two millennia, the only elements acknowledged were what I like to refer to as the "Captain Planet elements": earth, fire, air, and water.
Let’s take a moment to reflect on the absurdity of Captain Planet. Who decided that a superhero sporting a green mullet was a good representation of environmentalism? As a child, I found it perplexing, and I still question whether mullets were ever in vogue.
Now, let’s shift our minds back to a time before we learned about atoms, gravity, and cells—specifically to ancient Greece, where scientific inquiry often involved burning materials. This primitive analytical method allowed early scientists to derive conclusions about the composition of substances, setting the stage for our understanding today.
Section 1.1: The Primitive Science of Ancient Greece
In ancient Greece, the process of burning wood produced ash, which appeared to be an irreducible substance. The Greeks theorized that if one could reclaim the fire created, it could be combined with ash to recreate wood—a rudimentary form of the scientific method that persisted until the 17th century.
Consider the average laborer in ancient Greece: their daily life rarely intersected with an understanding of the chemical makeup of wine or oil. Knowledge of chemistry was likely only crucial for jewelers and metalworkers concerned with counterfeiting. Yet, despite this knowledge gap, Aristotle emerged as a pivotal figure in chemistry.
Subsection 1.1.1: Aristotle's Contributions to Chemistry
Despite his brilliance, Aristotle's approach to chemistry was not scientifically rigorous by today's standards. In fact, a modern high school student possesses more knowledge about elements than Aristotle ever did. His theories, which he adapted from Empedocles, dominated scientific thought for centuries, with little advancement in the understanding of matter until relatively recently.
For thousands of years, from Aristotle’s time through the rise and fall of various empires, the fundamentals of chemistry remained unchanged. His concept of elements derived more from philosophical contemplation than empirical experimentation. To Aristotle, an element represented the purest form of a substance, a notion that still holds some relevance in introductory chemistry courses today.
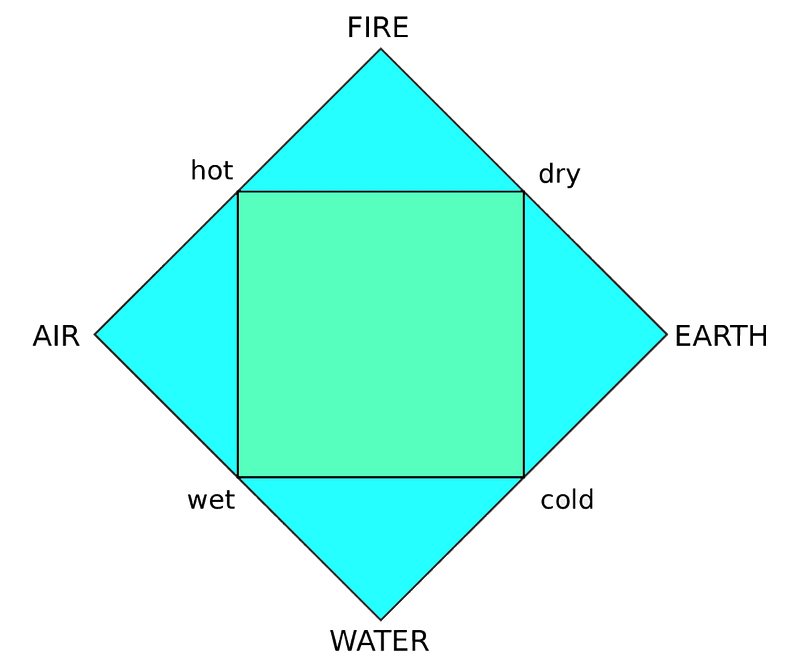
The diagram above illustrates the four classical elements, demonstrating how they were perceived through tangible observations—where ‘air’ combined with ‘water’ produced the feeling of wetness. This simplistic view of elements might seem appealing compared to our current complex periodic table.
Consider the medieval student preparing for a chemistry quiz: “With only four elements, this will be a breeze!”
Section 1.2: Understanding the Limitations of Classical Elements
Although notable elements like gold and silver had been identified well before Aristotle’s time, he categorized them based on philosophical reasoning. Gold, for instance, was deemed to belong to the earth element because it was extracted from the ground. The best experimentalists of his era, the alchemists, were more focused on transforming substances than on theorizing about their compositions.
As I delve into the history of chemistry, I often ponder the contributions of thinkers like Galileo, da Vinci, and Copernicus. It’s fascinating to consider how their insights could have enriched the study of chemistry, had they been afforded the opportunity.
The takeaway from this exploration is that for countless years, chemistry revolved around a mere four elements—elements that, in our modern understanding, aren’t even truly elements. It's a baffling thought that society could equate the brilliance of metallic gold with a simple classification of earth.
Next time, we’ll dive deeper into the evolution of chemical thought and the heroes of chemical manipulation, though they weren’t quite like the Planeteers.

Chapter 2: The Evolution of Chemical Thought
Exploring the brief history of chemistry, this video delves into how early theories shaped our current understanding of the elements.
This video offers insights into the historical development of chemistry, focusing on key figures and their contributions to the field.